Table of Contents |
guest 2025-12-25 |
Evaluating LabKey?
Demo Study
LabKey Studies
This research data portal highlights key features of LabKey's study module, which provides everything researchers need to manage their clinical and experimental data, including:
- Quality control and data curation
- Alignment of heterogeneous datasets, such as clinical, experimental, and sample data
- Visualizations, dashboards, and live reports
- Security and data de-identification for publishing
- and much more
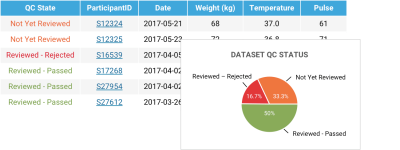
Data Quality ControlCustomize your own quality control states to apply to your data. Built-in quality control reports provide an overview of the status of all datasets and help you identify which data is in need of review and approval. |
 |
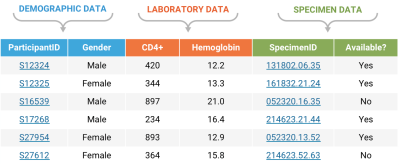
AlignmentLink all your datasets into an integrated whole for analysis and presentation. Experimental, demographic, and clinical datasets are automatically connected upon import to LabKey. Data from disparate sites can be easily connected using web-based data entry tools and drag-and-drop file upload. |
 |
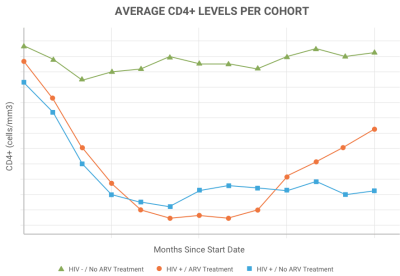
Visualization & AnalysisVisualize, analyze and display integrated study data using a range of built-in plotting and reporting tools available in LabKey Server. Use LabKey's built-in support for R to present live reports within a LabKey Server study. |
|
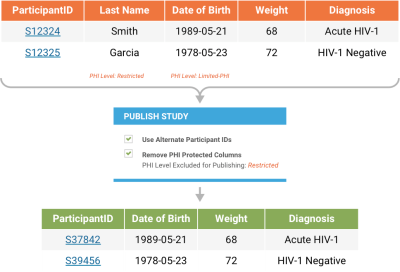
Publication and SecurityProvide direct access to study results using LabKey Server's data publishing tools. Provide access to a subset of data to a selected audience, such as collaborators or the general public. De-identify Protected Health Information (PHI) by randomizing participant IDs, shifting dates, and masking clinic names in published datasets. Or hold back specified columns of data from publication. These data de-identification features help support HIPAA compliant data sharing. |
 |
Case Studies
See LabKey's study management tools in action.
- Case Study #1: ITN Trial Share- A research data portal for both operational data management and publication of data from clinical trials.
- Case Study #2: LabKey Open Research Portal- A portal supporting multiple teams investigating emerging viruses such as Zika and Covid-19 (Coronavirus).
LabKey Studies: Learn More
- Clinical Research Software for Studies and Trials
- Data Sharing in Emerging Infectious Disease Research
- 3 Key Reasons Data Accessibility is Essential in Research