When you export a study, you can choose which items to include in the archive. In addition to queries, views, reports, and settings available when
exporting any type of folder, the study folder enables the export of study related properties.
- To export a study:
- From the Manage tab, click the Export Study button.
- Or select Admin > Folder > Management and click the Export tab.
- Select the checkboxes for folder and study objects you wish to export. The list will look something like the following:
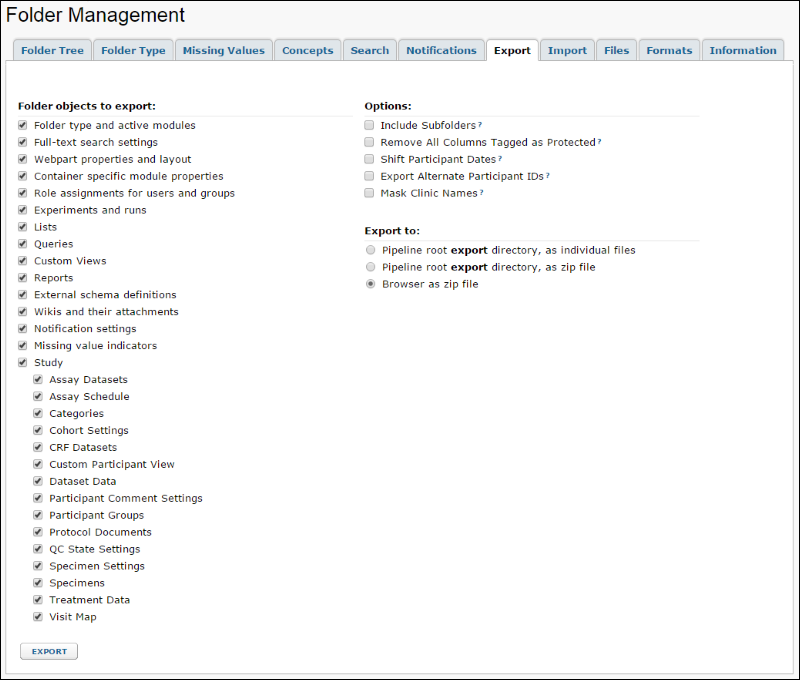
Assay Datasets
This option exports assay dataset information, writing metadata to "datasets_manifest.xml" and data to .tsv files. See
Study Import/Export Files and Formats for more details.
Assay Schedule
Exports
assay schedule .tsv files to a directory parallel to datasets in the archive including definitions of which assays are included in the study and expectations for which visits assay data will be uploaded.
Categories
This option exports the Categories for report grouping to "view_categories.xml."
Cohort Settings
This option exports the cohort definitions to "cohorts.xml." If defined, SubjectCount and Description for cohorts are included.
CRF Datasets
This option exports Case Report Form dataset information, writing meta data to "datasets_manifest.xml" and "datasets_metadata.xml" files and data to .tsv files. See
Study Import/Export Files and Formats for more details.
Custom Participant View
For a study where the admin has pasted in a custom Participant HTML page, the custom participant view is exported as participant.html.
Dataset Data
Export data in datasets; omitting this object creates a study template for use creating new matching but empty studies.
Participant Comment Settings
This option exports participant comment settings, if present.
Participant Groups
This option exports the study's participant groups. In addition to label, type, and datasetID, the autoUpdate attribute will record whether the group should be updated automatically. The file generated is "participant_groups.xml."
Protocol Documents
This option exports the study protocol documents to a "protocolDocs" folder.
QC State Settings
This option exports a "quality_control_states.xml" file that includes QC state definitions including custom states, descriptions, default states for the different import pathways and the default blank QC state.
Specimen Settings
This option exports a "specimen_settings.xml" file containing the groupings, location types, statuses, actors, and requirements you have defined. If you later import that archive into an existing specimen repository, any new specimen settings will be added. Any status or actor that is currently in use in the specimen repository will not be replaced from the imported archive. When you import an in-use actor, the membership emails for that actor will be replaced.
Note that there are some settings associated with specimen repositories which are not covered by this option. For example, custom properties defined for specimen tables are only exported in a full study archive.
For additional information about specimen repository settings and options, see
Specimens: Administrator Guide.
Specimens
This option exports a "specimens" directory containing the specimen archive itself as a .specimens file. For more about specimen archives, see
Specimen Archive File Reference. Note that this archive includes the data only - select export of specimen repository settings separately as described above.
Treatment Data
Include information about
study products and immunization treatments including immunogens, adjuvants, doses, and routes.
Visit Map
This option exports a "visit_map.xml" file detailing the baseline and visit schedule for the exported study.
More: For more information about export options and study schema, see
Study Import/Export Files and Formats.