Electronic signatures are designed to help your organization comply with the FDA Code of Federal Regulations (CFR) Title 21 Part 11.
Electronic signatures provide the following data validation mechanisms:
- Electronic signatures link the data to a reason for signing, the name of the signatory, and the date/time of the signature.
- Electronic signatures are linked to a unique and unrepeatable id number.
- Signatories must authenticate themselves with a username and password before signing.
Signing data resembles
exporting a data grid, except, when data is signed, the signatory is presented with a pop-up dialog where a reason for signing can be specified. Only the rows in a given data grid that have been selected will be signed.
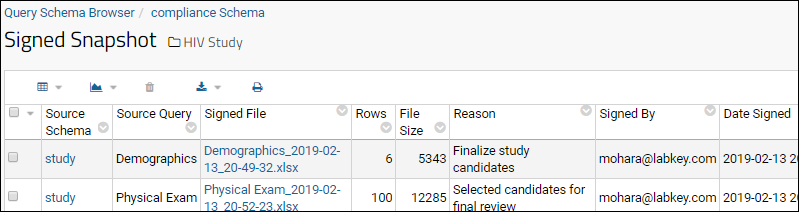
Upon signing, an Excel document of the signed data is created and added to the database as a record in the Signed Snapshot table. The Signed Snapshot table is available to administrators in the Query Browser in the compliance schema. You can add the
Signed Snapshot web part to a page to allow users to download signed documents. The record includes the following information:
- The Excel document (i.e., the signed data) is included as an attachment to the record
- The source schema of the signed document
- The source query of the signed document
- The number of rows in the document
- The file size of the document
- The reason for signing the document
- The signatory
- The date of signing
Set Up
The electronic signature functionality is part of the compliance module, which must be installed on the server and enabled in your folder before use. When enabled, all data grids that show the
or Export button can also be signed.
Select and Sign Data
To electronically sign data:
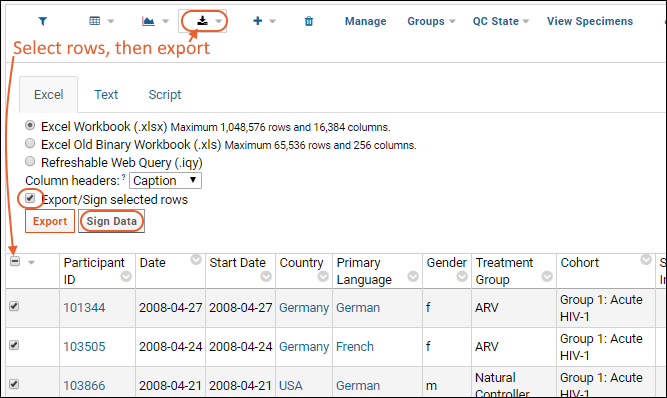
- Go to the data grid which includes the data you intend to sign.
- Select some or all of the rows from the data grid. Only rows that you select will be included in the signed document.
- Click (Export/Sign Data).
- On the Excel tab, confirm that Export/Sign selected rows is selected and click Sign Data.
- In the Sign Data Snapshot pop-up dialog, enter your username, password, and a reason for signing. (Note that if you already signed in to the server, you username and password will be pre-populated.)
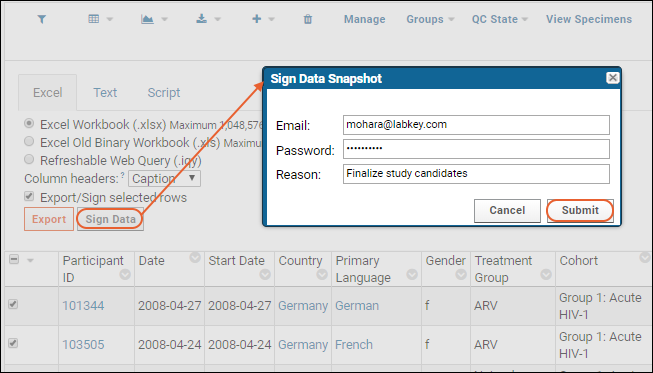
- Click Submit.
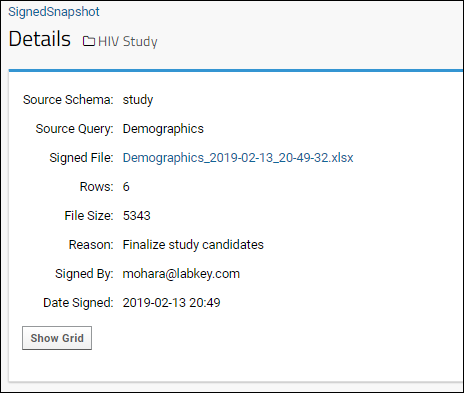
- Upon submission, you will be shown the details page for the record that has been inserted into the Signed Snapshot table.
Download Signed Data
To download a signed snapshot, view the Signed Snapshot table via the Schema Browser.
- Select (Admin) > Go To Module > Query.
- Select the compliance schema, and click the Signed Snapshot table to open it.
- Click View Data to see the snapshots.
- An administrator may create a "Query" web part to broaden access to such snapshots to users without schema browser access to this table.
- To download, click the name of the desired signed file. All downloads are audited.
Metadata Included in Exported Document
Signature metadata (the signatory, the source schema, the unique snapshot id, etc.) is included when you export the signed document. Metadata can be found in the following locations, depending on the download format:
| Downloaded Format | Metadata Location |
|---|
| Text format (TSV, CSV, etc) | The signature metadata is included in the first rows of the document as comments. |
| Excel format (XLS, XLSX) | The signature metadata is included in the document properties.
On Windows, go to File > Info > Properties > Advanced Properties.
On Mac, go to File > Properties > Custom. |
Auditing Electronic Signatures
Electronic signature events are captured in the audit log.
- Select (Admin) > Site > Admin Console.
- Under Management click Audit Log.
- From the dropdown, select Signed snapshots.
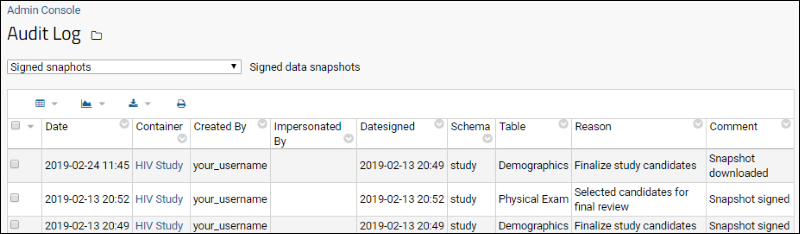
The audit log captures:
- The user who signed, i.e. created the signed snapshot
- The date and time of the event (signing, downloading, or deletion of a signature)
- The date and time of the signature itself
- The container (folder) and schema in which the signed snapshot resides
- The table that was signed
- The reason for signing
- A comment field where the type of event is described:
- Snapshot signed
- Snapshot downloaded: The date of download and user who downloaded it are recorded
- Snapshot deleted: The user who deleted the signed snapshot is recorded.
Related Topics



